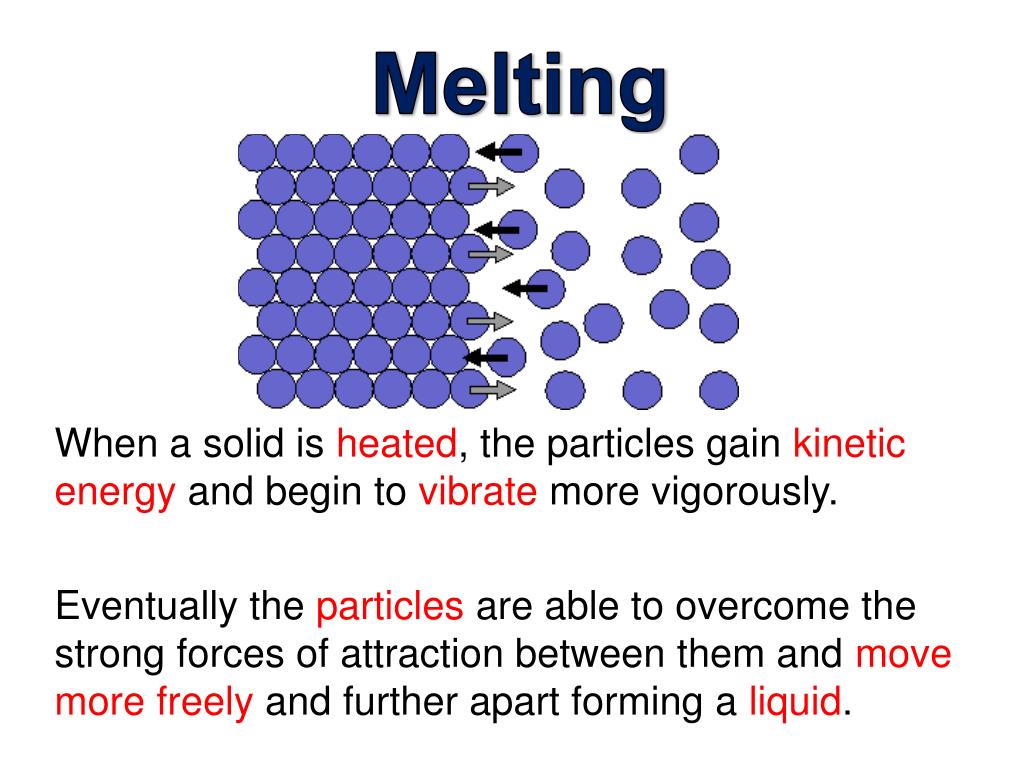
What Happens When Ice Is Heated And Melts To Form Water . when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. the ice placed in the water will melt faster than the ice in air. when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: melting of ice occurs in two steps: after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Since the water and the air are both at room temperature, it. The hexagonal channels become partially filled, and the. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;.
from www.slideserve.com
First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;. Since the water and the air are both at room temperature, it. the ice placed in the water will melt faster than the ice in air. when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. melting of ice occurs in two steps: The hexagonal channels become partially filled, and the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise.
PPT The Nature of Matter PowerPoint Presentation, free download ID
What Happens When Ice Is Heated And Melts To Form Water after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;. melting of ice occurs in two steps: when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. the ice placed in the water will melt faster than the ice in air. Since the water and the air are both at room temperature, it. The hexagonal channels become partially filled, and the.
From classroom.synonym.com
What Happens When Ice Is Added to Hot Water and How Will the Energy What Happens When Ice Is Heated And Melts To Form Water when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. Since the water and the air are both at room temperature, it. when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. the ice placed in the water will. What Happens When Ice Is Heated And Melts To Form Water.
From www.fity.club
Melting Ice Cubes Diagram What Happens When Ice Is Heated And Melts To Form Water melting of ice occurs in two steps: The hexagonal channels become partially filled, and the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: First, the phase change occurs and solid (ice) transforms into liquid water at the melting. What Happens When Ice Is Heated And Melts To Form Water.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, What Happens When Ice Is Heated And Melts To Form Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. after the ice has completely melted, continued heating of the water will. What Happens When Ice Is Heated And Melts To Form Water.
From ar.inspiredpencil.com
Melting Point Of Ice What Happens When Ice Is Heated And Melts To Form Water when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: The hexagonal channels become partially filled, and the. First, the phase change occurs. What Happens When Ice Is Heated And Melts To Form Water.
From exotpvkkb.blob.core.windows.net
Hot Salt Water Melt Ice at Eddie Combs blog What Happens When Ice Is Heated And Melts To Form Water when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. Since the water and the air are both at room temperature, it. after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will,. What Happens When Ice Is Heated And Melts To Form Water.
From exojpxgyg.blob.core.windows.net
When Ice Melts What Happens To The Water Molecules at Marjorie Alm blog What Happens When Ice Is Heated And Melts To Form Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. melting of ice occurs in two steps: First,. What Happens When Ice Is Heated And Melts To Form Water.
From www.youtube.com
Final Temperature of Ice and Water Mixture How Many Grams of Ice Will What Happens When Ice Is Heated And Melts To Form Water when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. Since the water and the air are both at room temperature, it. after the ice has completely melted, continued. What Happens When Ice Is Heated And Melts To Form Water.
From smartclass4kids.com
Changing States of Matter Solid, Liquid,Gas, Phase Change What Happens When Ice Is Heated And Melts To Form Water this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: melting of ice occurs in two steps: when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. . What Happens When Ice Is Heated And Melts To Form Water.
From www.learnz.org.nz
The Water Cycle LEARNZ What Happens When Ice Is Heated And Melts To Form Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;. The hexagonal channels become partially filled, and the. the ice placed in the water will melt faster than the ice in air. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c. What Happens When Ice Is Heated And Melts To Form Water.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science What Happens When Ice Is Heated And Melts To Form Water melting of ice occurs in two steps: Since the water and the air are both at room temperature, it. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: the ice placed in the water will melt faster than. What Happens When Ice Is Heated And Melts To Form Water.
From quizlet.com
Phases of Matter and Heat Diagram Quizlet What Happens When Ice Is Heated And Melts To Form Water The hexagonal channels become partially filled, and the. when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. the ice placed in the water will melt faster than the ice in air. First, the phase change occurs and solid (ice) transforms into liquid water at the melting. What Happens When Ice Is Heated And Melts To Form Water.
From sciencing.com
What Happens to the Temperature of Ice As it Melts? Sciencing What Happens When Ice Is Heated And Melts To Form Water after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Since the water and the air are both at room temperature, it. melting of ice occurs in two steps: The hexagonal channels become partially filled, and the. when ice. What Happens When Ice Is Heated And Melts To Form Water.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid What Happens When Ice Is Heated And Melts To Form Water after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. Since the water and the air are both at room temperature, it. First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;. when an. What Happens When Ice Is Heated And Melts To Form Water.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID What Happens When Ice Is Heated And Melts To Form Water after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: melting. What Happens When Ice Is Heated And Melts To Form Water.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office What Happens When Ice Is Heated And Melts To Form Water melting of ice occurs in two steps: The hexagonal channels become partially filled, and the. after the ice has completely melted, continued heating of the water will now increase the kinetic energy of the liquid molecules and the temperature will, once again, rise. the ice placed in the water will melt faster than the ice in air.. What Happens When Ice Is Heated And Melts To Form Water.
From www.slideshare.net
Heat What Happens When Ice Is Heated And Melts To Form Water First, the phase change occurs and solid (ice) transforms into liquid water at the melting temperature;. when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as. What Happens When Ice Is Heated And Melts To Form Water.
From phys.org
Scientists characterize the phase transitions of melting ice layers What Happens When Ice Is Heated And Melts To Form Water when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hexagonal channels become partially filled, and the. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: First, the phase change occurs. What Happens When Ice Is Heated And Melts To Form Water.
From www.iberdrola.com
Melting Glaciers causes, effects and solutions Iberdrola What Happens When Ice Is Heated And Melts To Form Water when an ice cube is heated, it melts, and if the liquid water continues to be heated it will eventually boil. Since the water and the air are both at room temperature, it. when ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. this plot of temperature shows what happens. What Happens When Ice Is Heated And Melts To Form Water.